Importance Of Serial Dilution In Serology Tests
- Importance Of Serial Dilution In Serology Tests For Adults
- Importance Of Serial Dilution In Serology Tests 1
Serological tests. 1.
Chapter 8: Serology Laboratory Math. The characteristics of the assay, the sensitivity and specificity and on population characteristics in order to obtain clinical results most appropriate for diagnosis of intended target population, the assay with the characteristic most important to the diagnostic picture should be chosen yielding the BEST POSITIVE PREDICTIVE VALUE. A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. The easiest method is to make a series of 1 in 10 dilutions. Feb 11, 2015 - passive agglutination assays. Serial dilutions. Serologic testing has long been an important part of diagnostic tests in the clinical laboratory for viral and bacterial diseases. Immunologic testing is done in many areas of the clinical laboratory—microbiology,.
Serological tests(Antigen antibody interactions). Classification of antigen-antibodyinteractions:1. Primary serological tests: (Marker techniques) e.g.– Enzyme linked immuono sorben assay (ELISA)– Immuno flurescent antibody technique (IFAT)– Radio immuno assay (RIA)2. Secondary serological tests: e.g.– Agglutination tests– Complement fixation tests (CFT)– Precipitation tests– Serum neutralization tests (SNT)– Toxin-antitoxin test3. Tertiary serological test: e.g.– Determination of the protective value of an anti serum in an animal. Agglutination tests:1.
Agglutination/HemagglutinationWhen the antigen is particulate, the reaction of an antibody with theantigen can be detected by agglutination (clumping) of the antigen.The general term agglutinin is used to describe antibodies thatagglutinate particulate antigens. When the antigen is an erythrocytethe term heamagglutination is used.
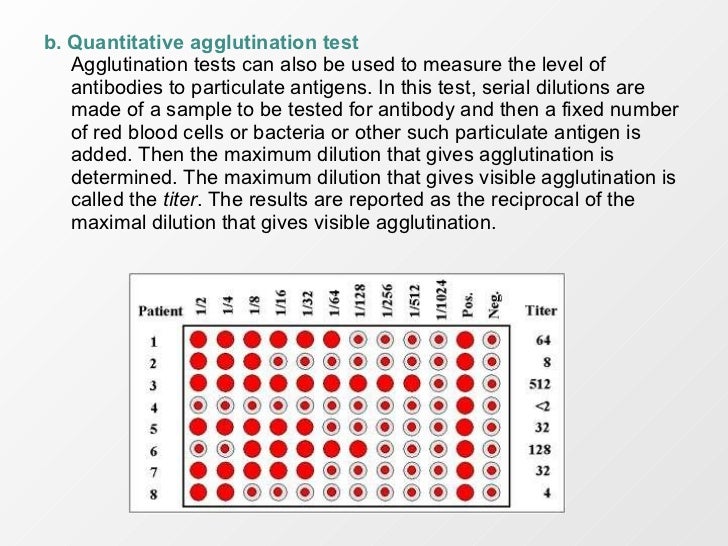
All antibodies can theoreticallyagglutinate particulate antigens but IgM, due to its high valence, isparticularly good agglutinin and one sometimes infers that anantibody may be of the IgM class if it is a good agglutinatingantibody.a. Qualitative agglutination testAgglutination tests can be used in a qualitative manner to assay forthe presence of an antigen or an antibody. The antibody is mixedwith the particulate antigen and a positive test is indicated by theagglutination of the particulate antigen. For example, a patients red blood cells can be mixed with antibody to ablood group antigen to determine a persons blood type. In a secondexample, a patients serum is mixed with red blood cells of a knownblood type to assay for the presence of antibodies to that blood typein the patients serum. Quantitative agglutination testAgglutination tests can also be used to measure the level ofantibodies to particulate antigens. In this test, serial dilutions aremade of a sample to be tested for antibody and then a fixed numberof red blood cells or bacteria or other such particulate antigen isadded.
Importance Of Serial Dilution In Serology Tests For Adults
Then the maximum dilution that gives agglutination isdetermined. The maximum dilution that gives visible agglutination iscalled the titer. Rotary vacuum filter pdf. The results are reported as the reciprocal of themaximal dilution that gives visible agglutination. Prozone effect - Occasionally, it is observed that when theconcentration of antibody is high (i.e. Lower dilutions), there is noagglutination and then, as the sample is diluted, agglutinationoccurs.The lack of agglutination at high concentrations of antibodies is calledthe prozone effect. Lack of agglutination in the prozone is due toantibody excess resulting in very small complexes that do not clumpto form visible agglutination.c.
Applications of agglutination testsi. Determination of blood types or antibodies to bloodgroup antigens.ii.
To assess bacterial infectionse.g. A rise in titer of an antibody to a particular bacteriumindicates an infection with that bacterial type. Afourfold rise in titer is generally taken as a significant risein antibody titer. 2-Passive hemagglutination:The agglutination test only works with particulate antigens.However, it is possible to coat erythrocytes with a soluble antigen(e.g. Viral antigen, a polysaccharide or a hapten) and use the coatedred blood cells in an agglutination test for antibody to the solubleantigen. This is called passive hemagglutination.The test is performed just like the agglutination test. Applicationsinclude detection of antibodies to soluble antigens and detection ofantibodies to viral antigens.
3-Coombs Test (Antiglobulin Test):a. Direct Coombs TestWhen antibodies bind to erythrocytes, they do not always result inagglutination. This can result from the antigen/antibody ratio being inantigen excess or antibody excess or in some cases electrical chargeson the red blood cells preventing the effective cross linking of the cells.These antibodies that bind to but do not cause agglutination of red bloodcells are sometimes referred to as incomplete antibodies. In no way isthis meant to indicate that the antibodies are different in their structure,although this was once thought to be the case. Rather, it is a functionaldefinition only. In order to detect the presence of non-agglutinatingantibodies on red blood cells, one simply adds a second antibodydirected against the immunoglobulin (antibody) coating the red cells. Thisanti-immunoglobulin can now cross link the red blood cells and result inagglutination.b.
Indirect Coombs TestIf it is necessary to know whether a serum sample has antibodiesdirected against a particular red blood cell and you want to be sure thatyou also detect potential non- agglutinating antibodies in the sample, anIndirect Coombs test is performed.This test is done by incubating the red blood cells with the serum sample,washing out any unbound antibodies and then adding a second anti-immunoglobulin reagent to cross link the cells. ApplicationsThese include detection of anti-rhesus factor (Rh) antibodies.
Antibodies tothe Rh factor generally do not agglutinate red blood cells. Thus, red cellsfrom Rh+ children born to Rh- mothers, who have anti-Rh antibodies, maybe coated with these antibodies. To check for this, a direct Coombs test isperformed. To see if the mother has anti-Rh antibodies in her serum anIndirect Coombs test is performed. 4-Hemagglutination InhibitionThe agglutination test can be modified to be used for the measurement ofsoluble antigens. This test is called hemagglutination inhibition. It is calledhemagglutination inhibition because one measures the ability of solubleantigen to inhibit the agglutination of antigen-coated red blood cells byantibodies.
In this test, a fixed amount of antibodies to the antigen inquestion is mixed with a fixed amount of red blood cells coated with theantigen. Also included in the mixture are different amounts of the sample tobe analyzed for the presence of the antigen. If the sample contains theantigen, the soluble antigen will compete with the antigen coated on the redblood cells for binding to the antibodies, thereby inhibiting the agglutinationof the red blood cells. Precipitation tests1-Radial Immunodiffusion (Mancini)In radial immunodiffusion antibody is incorporated into the agar gel as it ispoured and different dilutions of the antigen are placed in holes punchedinto the agar. As the antigen diffuses into the gel, it reacts with the antibodyand when the equivalence point is reached a ring of precipitation is formed.2-Immunoelectrophoresis:In immunoelectrophoresis, a complex mixture of antigens is placed in a wellpunched out of an agar gel and the antigens are electrophoresed so that theantigen are separated according to their charge. After electrophoresis, atrough is cut in the gel and antibodies are added.
As the antibodies diffuseinto the agar, precipitin lines are produced in the equivalence zone when anantigen/antibody reaction occurs. This tests is used for the qualitative analysis of complex mixtures of antigens,although a crude measure of quantity (thickness of the line) can beobtained. This test is commonly used for the analysis of components in apatient serum. Serum is placed in the well and antibody to whole serum inthe trough. By comparisons to normal serum, one can determine whetherthere are deficiencies on one or more serum components or whether thereis an overabundance of some serum component (thickness of the line). Thistest can also be used to evaluate purity of isolated serum proteins. 3-Countercurrent electrophoresis:In this test the antigen and antibody are placed in wellspunched out of an agar gel and the antigen and antibodyare electrophoresed into each other where they form aprecipitation line.This test only works if conditions can be found where theantigen and antibody have opposite charges.
This test isprimarily qualitative, although from the thickness of theband you can get some measure of quantity. Its majoradvantage is its speed. Complement fixation test. The complement fixation test is an immunologicalmedical test looking for evidence of infection.
It tests forthe presence of either specific antibody or specificantigen in a patients serum. It uses sheep red bloodcells (sRBC), anti-sRBC antibody and complement, plusspecific antigen (if looking for antibody in serum) orspecific antibody (if looking for antigen in serum). If either the antibody or antigen is present in the patientsserum, then the complement is completely utilized, sothe sRBCs are not lysed. But if the antibody (or antigen)is not present, then the complement is not used up, so itbinds anti-sRBC antibody, and the sRBCs are lysed. The Wassermann test is one form of complementfixation test.
Enzyme-Linked ImmunoSorbent Assay (ELISA). Enzyme-Linked ImmunoSorbent Assay, or ELISA, is abiochemical technique used mainly in immunology todetect the presence of an antibody or an antigen in asample. The ELISA has been used as a diagnostic toolin medicine and plant pathology, as well as a qualitycontrol check in various industries.
In simple terms, inELISA an unknown amount of antigen is affixed to asurface, and then a specific antibody is washed over thesurface so that it can bind the antigen. This antibody islinked to an enzyme, and in the final step a substance isadded that the enzyme can convert to some detectablesignal. Thus in the case of fluorescence ELISA, whenlight is shone upon the sample, any antigen/antibodycomplexes will fluoresce so that the amount of antigen inthe sample can be measured. Indirect ELISA. The steps of the general, 'indirect,' ELISA for determining serumantibody concentrations are:1. Apply a sample of known antigen of known concentration to asurface, often the well of a microtiter plate. The antigen is fixed to thesurface to render it immobile.
Simple adsorption of the protein to theplastic surface is usually sufficient. These samples of known antigenconcentrations will constitute a standard curve used to calculateantigen concentrations of unknown samples. Note that the antigenitself may be an antibody.2.
The plate wells or other surface are then coated with serum samplesof unknown antigen concentration, diluted into the same buffer usedfor the antigen standards. Since antigen immobilization in this step isdue to non-specific adsorption, it is important for the total proteinconcentration to be similar to that of the antigen standards.3. A concentrated solution of non-interacting protein, such as BovineSerum Albumin (BSA) or casein, is added to all plate wells.
This stepis known as blocking, because the serum proteins block non-specificadsorption of other proteins to the plate. The plate is washed, and a detection antibody specific to theantigen of interest is applied to all plate wells. This antibodywill only bind to immobilized antigen on the well surface, not toother serum proteins or the blocking proteins.5. The plate is washed to remove any unbound detectionantibody.
After this wash, only the antibody-antigen complexesremain attached to the well.6. Secondary antibodies, which will bind to any remainingdetection antibodies, are added to the wells. These secondaryantibodies are conjugated to the substrate-specific enzyme.This step may be skipped if the detection antibody isconjugated to an enzyme.7. Wash the plate, so that excess unbound enzyme-antibodyconjugates are removed.8. Apply a substrate which is converted by the enzyme to elicit achromogenic or fluorogenic or electrochemical signal.9.
View/quantify the result using a spectrophotometer,spectrofluorometer, or other optical/electrochemical device. To detect antibody (indirect ELISA):. 2.
Sandwich ELISA. A sandwich ELISA: Plate is coated with a capture antibody sample is added, and any antigen presentbinds to capture antibody detecting antibody is added, and binds toantigen enzyme-linked secondary antibody is added,and binds to detecting antibody substrate is added, and is converted byenzyme to detectable form. A less-common variant of this technique, called 'sandwich' ELISA, isused to detect sample antigen. The steps are as follows:1.
Prepare a surface to which a known quantity of capture antibodyis bound.2. Block any non specific binding sites on the surface.3.
Apply the antigen-containing sample to the plate.4. Wash the plate, so that unbound antigen is removed.5. Apply primary antibodies that bind specfically to the antigen.6. Apply enzyme-linked secondary antibodies which are specific tothe primary antibodies.7. Wash the plate, so that the unbound antibody-enzyme conjugatesare removed.8. Apply a chemical which is converted by the enzyme into a color orfluorescent or electrochemical signal.9.
Measure the absorbance or fluorescence or electrochemicalsignal (e.g., current) of the plate wells to determine the presenceand quantity of antigen. To detect antigen (sandwich ELISA):. 3. Competitive ELISA. A third use of ELISA is through competitive binding.The steps for this ELISA are somewhat different thanthe first two examples:1. Unlabeled antibody is incubated in the presence of its antigen.2.
These bound antibody/antigen complexes are then added toan antigen coated well.3. The plate is washed, so that unbound antibody is removed.(The more antigen in the sample, the less antibody will be ableto bind to the antigen in the well, hence 'competition.' The secondary antibody, specific to the primary antibody isadded. This second antibody is coupled to the enzyme.5.
A substrate is added, and remaining enzymes elicit achromogenic or fluorescent signal. For competitive ELISA, the higher the original antigenconcentration, the weaker the eventual signal. Competitive binding.
Applications. Because the ELISA can be performed to evaluate either the presence ofantigen or the presence of antibody in a sample, it is a useful tool both fordetermining serum antibody concentrations (such as with the HIV test 1 orWest Nile Virus) and also for detecting the presence of antigen.
It has alsofound applications in the food industry in detecting potential food allergenssuch as milk,peanuts,walnuts,almonds, and eggs 2The ELISA test, or theenzyme immunoassay (EIA), was the first screening test commonlyemployed for HIV. It has a high sensitivity.In an ELISA test, a personsserum is diluted 400-fold and applied to a plate to which HIV antigens havebeen attached. If antibodies to HIV are present in the serum, they may bindto these HIV antigens. The plate is then washed to remove all othercomponents of the serum. A specially prepared 'secondary antibody' — anantibody that binds to human antibodies — is then applied to the plate,followed by another wash.

This secondary antibody is chemically linked inadvance to an enzyme. Thus the plate will contain enzyme in proportion tothe amount of secondary antibody bound to the plate. A substrate for theenzyme is applied, and catalysis by the enzyme leads to a change in coloror fluorescence.
Importance Of Serial Dilution In Serology Tests 1
ELISA results are reported as a number; the mostcontroversial aspect of this test is determining the 'cut-off' point between apositive and negative result.
Comments are closed.